CSSD Center
Central Sterile Supply Department
Explore WEGO’s full portfolio of CSSD solutions—from sterilization wraps and containers to tracking indicators and washer accessories—engineered to support reliable reprocessing workflows and enhance patient safety.
WEGO Medical is committed in developing, manufacturing and supplying high quality, safe and reliable CSSD solution system that improve infection control, optimize processes and productivity, while minimizing costs.
Through our vast experience designing disinfection and sterilization and cutting-edge technology, our greatest aim is to develop high-quality solutions to prevent infection and cross contamination in healthcare facilities.
As a famous infection control solution provider, WEGO is a leader and pioneer company in the design and manufacture of Steam Sterilizer,
Low Temperature Plasma Sterilizer, Washer-disinfector, Medical Drying Cabinet, Ultrasonic Washer, Water treatment system, CSSD logistic &
warehousing system and Accessories, Consumables & Packing material and so on.


Advanced Soiled Goods Area
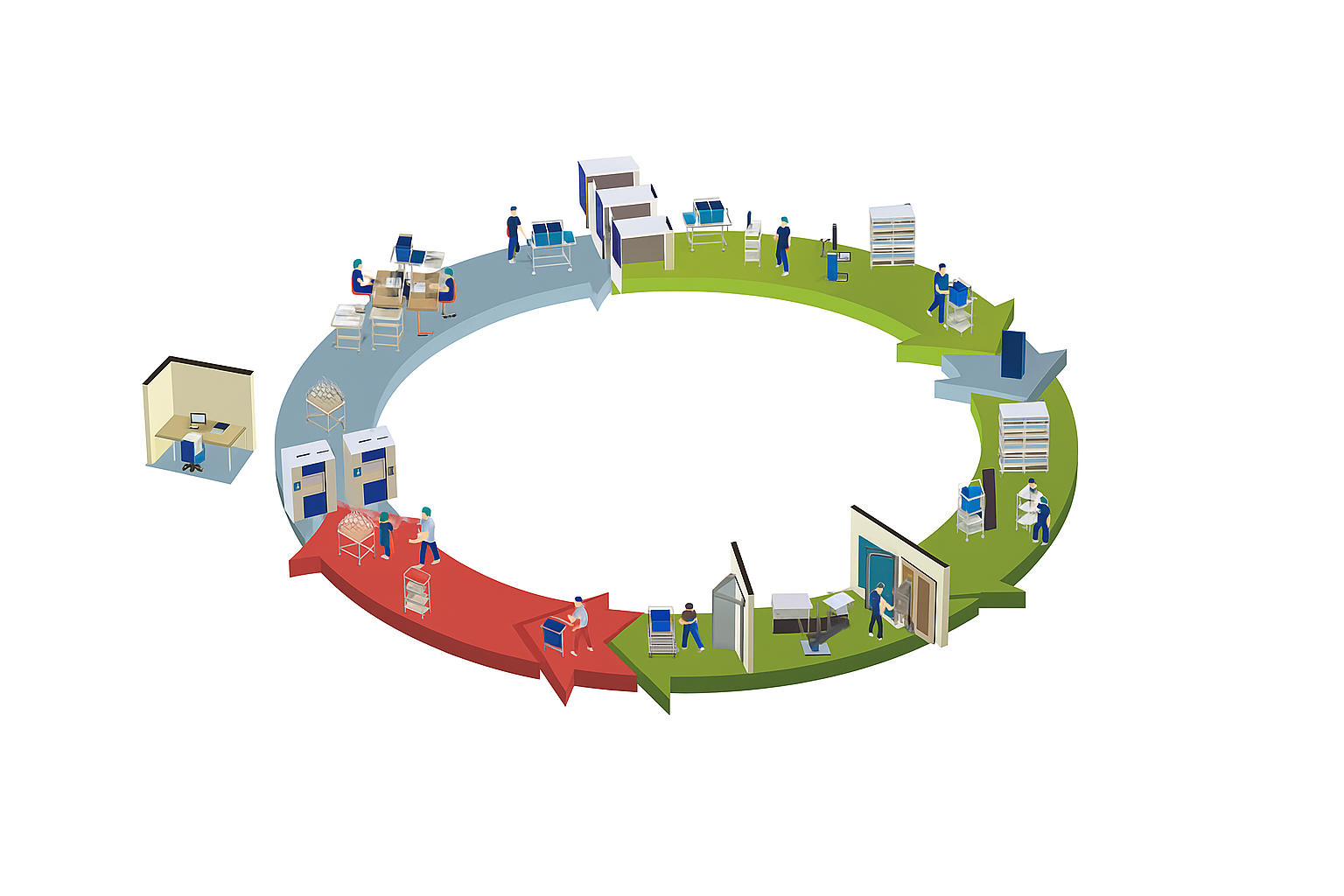
The Soiled Goods Area is where the first step of infection control begins. Contaminated instruments from operating rooms and wards are handled with precision using manual cleaning workstations for delicate tasks, ultrasonic washers for fine lumen instruments, and vacuum ultrasonic washer-disinfectors for complex devices. Larger volumes are efficiently processed through automatic washer-disinfectors, supported by a dedicated water treatment system to guarantee consistent cleaning quality. Finally, medical drying cabinets ensure that every instrument leaves this area dry, safe, and ready for further processing.
- View the Soiled Goods Area System>>


Reliable Sterile Area
The Sterile Area represents the final safeguard before instruments return to clinical use. After sterilization in advanced pressure steam sterilizers, items are securely managed within sterile storage systems that protect their integrity until use. Distribution is made safe and efficient with logistic trolleys, while a full range of consumables and accessories—from sealing machines to sterilization monitoring tools—supports smooth operation. Real-time scanning and inventory tracking guarantee that every sterile supply is reliable, traceable, and readily available for patient care.
- View the Sterile Goods Area System>>
Efficient Clean and Disinfection Area
The Clean and Disinfection Area offers a controlled environment where instruments are dried, inspected, assembled, and packaged with care. Medical drying cabinets create optimal conditions for safe handling, while automated packing systems and barcode labeling solutions streamline workflow and enhance traceability. Once ready, instruments move seamlessly into low-temperature plasma sterilizers for heat-sensitive devices or high-pressure steam sterilizers for standard surgical tools. This integrated process ensures thorough sterilization preparation, minimizes cross-contamination risks, and supports the highest hospital safety standards.
- View the Efficient Clean and Disinfection Goods Area System>>


Comprehensive Support from the WEGO Global Team
When you partner with WEGO, you gain more than trusted medical products – you gain the strength of the WEGO Global Support Team. We are dedicated to helping you maximize performance, ensure reliability, and deliver better outcomes for patients worldwide.
With 37 years of experience, advanced manufacturing, and a worldwide service network, WEGO provides timely assistance, technical guidance, and tailored solutions. Our team works side by side with you to keep operations running smoothly and efficiently.
At WEGO, our commitment starts before the first delivery and grows throughout our journey together. Your success drives our mission, and your trust fuels our progress.
- Access WEGO Expert Support >>

Q1. What is the role of CSSD in a hospital?
A: The Central Sterile Supply Department (CSSD) is responsible for cleaning, disinfecting, sterilizing, storing, and distributing medical instruments. It ensures infection control, patient safety, and workflow efficiency across all clinical departments.
Q2. Why is water treatment critical in CSSD?
A: Proper water quality (soft water or RO/DI water) prevents scale, reduces stains on instruments, enhances cleaning consistency, and prolongs equipment lifespan, ensuring compliance with international standards.
Q3. How to choose between plasma and steam sterilization?
A: Plasma sterilization is recommended for heat- and moisture-sensitive items, offering fast, low-temperature cycles with minimal residue. Steam sterilization is suitable for robust instruments that can withstand high temperature and pressure, ensuring broad-spectrum sterilization.
Q4. What accessories and consumables are required for daily CSSD operations?
A: Essential items include washing baskets, instrument trays, sealing machines, chemical and biological indicators, packaging materials, transport trolleys, and labeling systems. These ensure smooth workflow and monitoring of sterilization quality.
Q5. How is traceability achieved in CSSD?
A: Traceability is managed through barcode scanning or RFID tracking at each step (receiving, cleaning, packaging, sterilization, storage, distribution). This creates a complete digital record for compliance, recalls, and quality assurance.