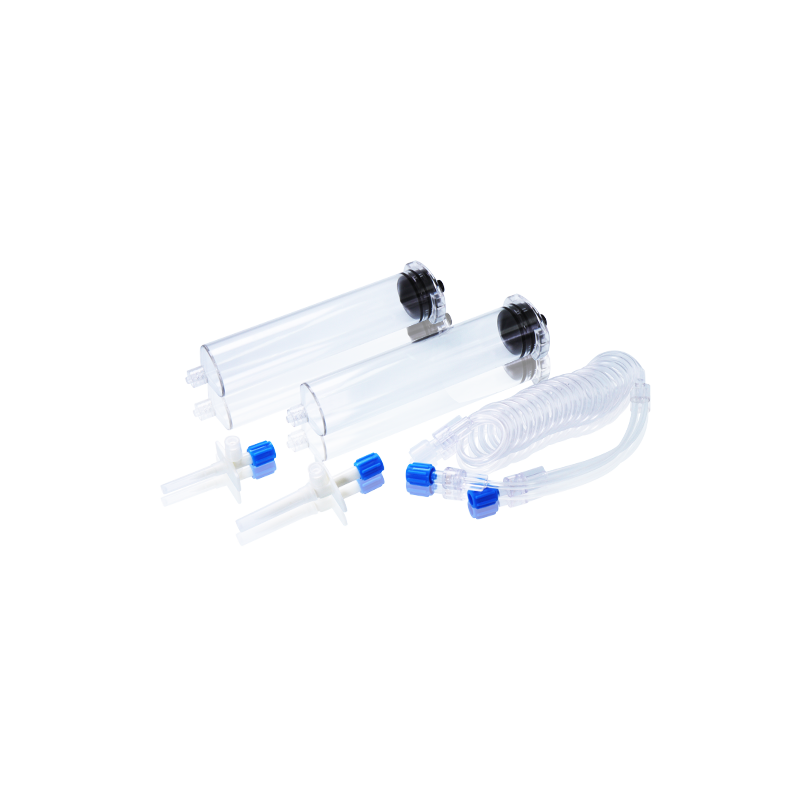
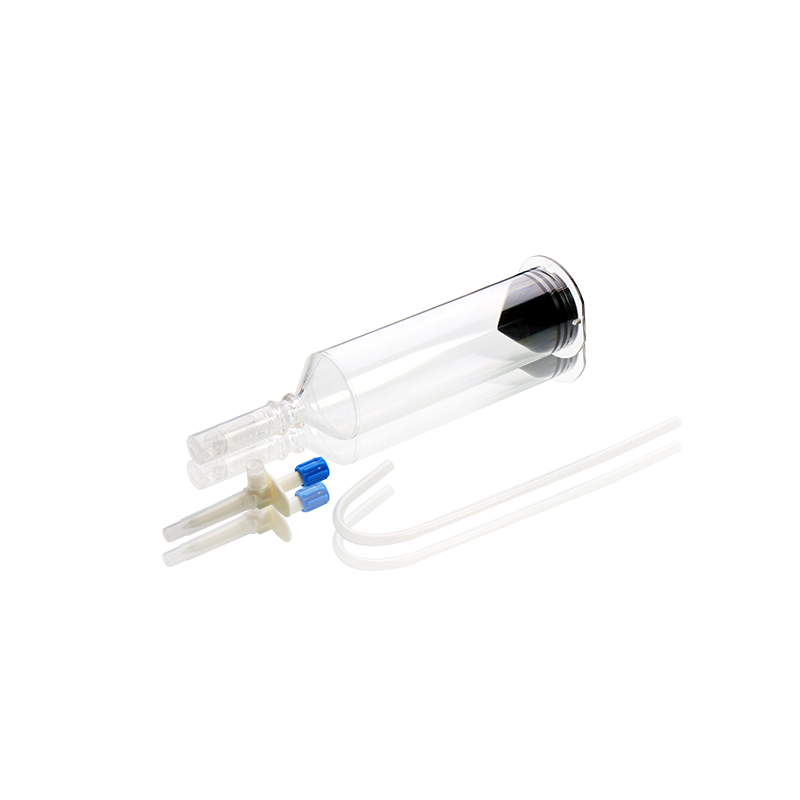
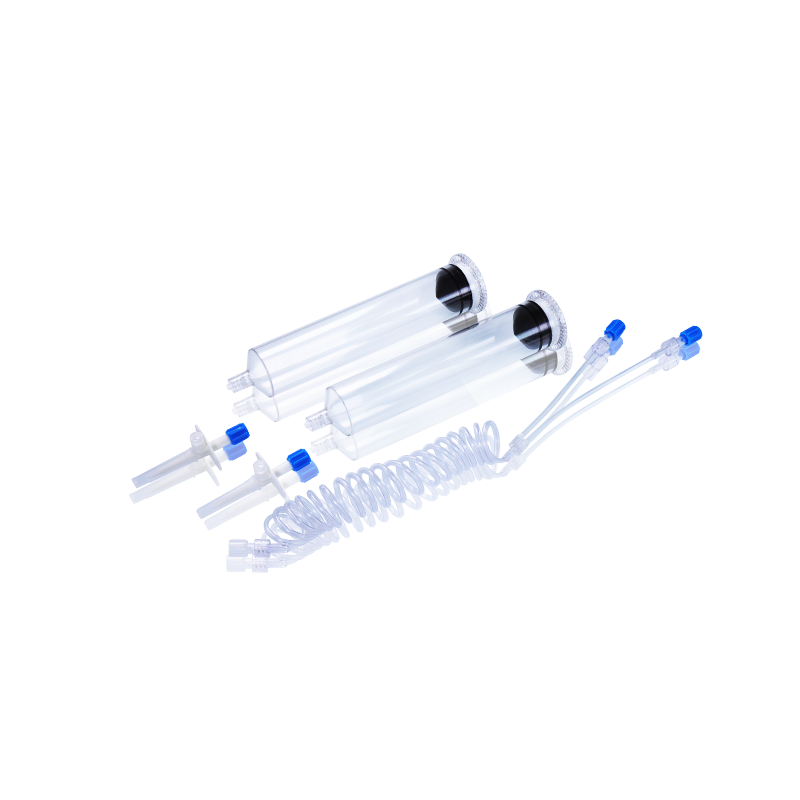
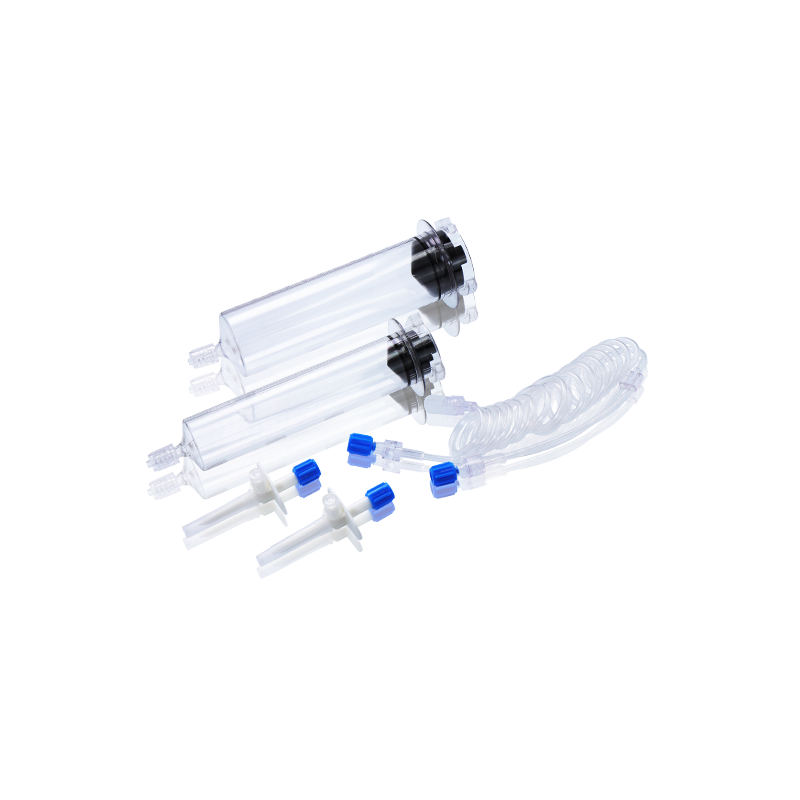
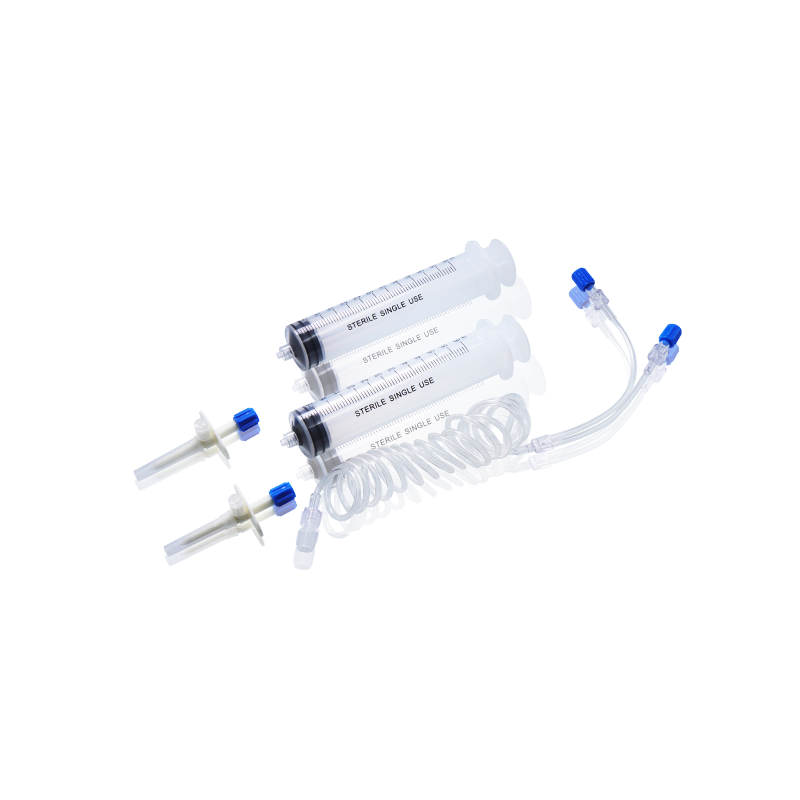
CT, MRI & DSA
Contrast Media Injector Syringes
Discover WEGO’s full range of disposable high-pressure syringes designed to meet the demanding needs of modern radiology and interventional imaging.
As a leading medical device manufacturer, WEGO Medical has been dedicated to developing high-quality disposable medical products for more than three decades. With a global presence and ISO 13485–certified production facilities, WEGO provides a comprehensive portfolio of solutions for clinical use, including infusion therapy, blood management, and diagnostic imaging.
Our disposable contrast media injector syringes are fully compatible with a wide range of injector systems used in hospitals, imaging centers, and radiology departments worldwide. Whether for CT, MRI, or DSA, WEGO syringes offer excellent adaptability, helping healthcare professionals achieve clearer imaging results and more efficient workflow.
Choose WEGO for dependable contrast injection solutions backed by decades of medical manufacturing expertise. With a global distribution network and continuous innovation, WEGO ensures safer, smarter, and more precise imaging for healthcare providers and patients around the world.


Clean Production
Produced in a Class 100,000 (ISO 8) cleanroom with constant temperature and humidity control.
Regular monitoring of settle plates, airborne particulates, and microbial flora ensures minimal bioburden for greater product safety and reliability.
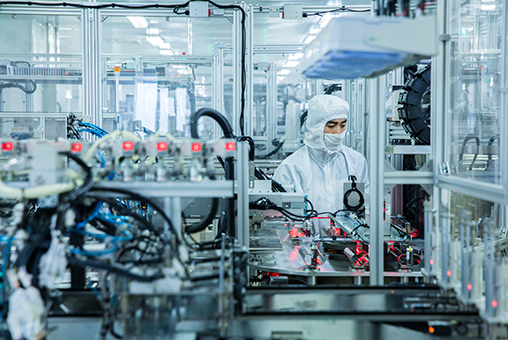
Precision Manufacturing
Equipped with advanced injection molding machines, high-precision hot runner molds, and temperature control systems.
The syringe barrel is siliconized with imported medical-grade silicone oil to reduce friction and extend injector life.
Made from high-grade PET material, it provides excellent transparency and minimal color deviation for easy visual inspection.
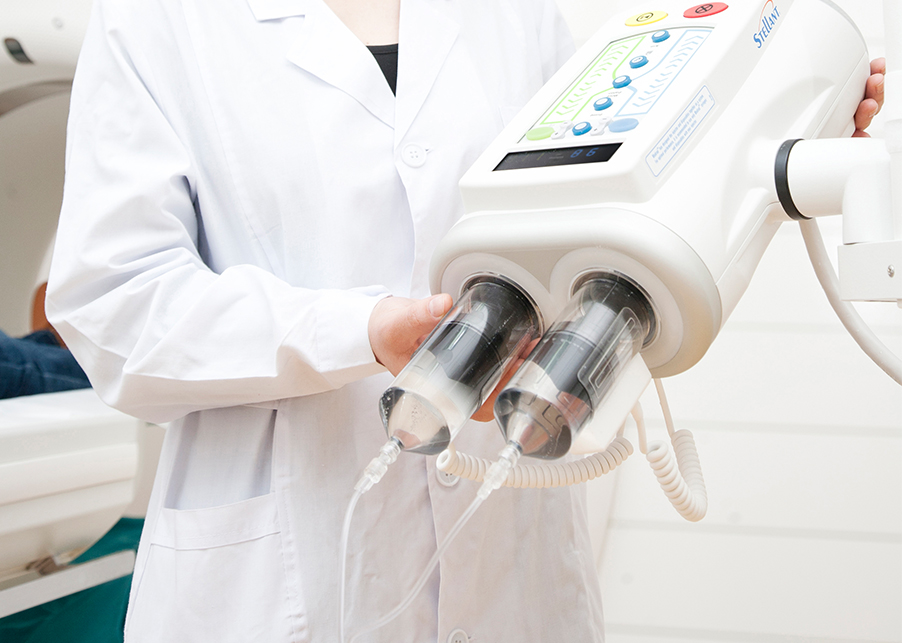
Safe & Versatile
Sterilized with ethylene oxide (EO) to ensure sterility and pyrogen-free status, preventing adverse reactions such as fever or phlebitis.
Available in multiple specifications compatible with CT, DSA, and MRI systems for flexible clinical use.

Q1. What should we do if there is high injection resistance or the device fails to recognize the syringe?
A:
High resistance: WEGO syringes are siliconized with imported silicone oil to reduce friction. If resistance persists, check for twisted connectors (e.g., conical tip may need one full turn to release).
Device not recognizing syringe: This may be due to parameter deviation or sensor malfunction. Please contact the equipment engineer for calibration.
Q2. How does WEGO ensure the sterility and safety of its contrast media syringes?
A:
The syringes are manufactured in a Class 100,000 cleanroom environment with strict temperature and humidity control. Regular monitoring is conducted for settling bacteria, airborne particles, and microorganisms.
An ethylene oxide (EO) sterilization process is used to ensure the products are sterile and pyrogen-free, minimizing the risk of pyrogenic reactions and phlebitis.
Imported raw materials and fully automated production lines are employed to reduce the risk of human contamination.
Q3. Do the syringes meet the pressure requirements of different imaging devices?
A:
All syringes are reinforced with screw-type connectors to enhance pressure resistance and reduce leakage risk.
WEGO offers dedicated syringes tailored to various imaging systems with clinically matched pressure resistance:
CT series: Up to 300–600 psi (e.g., 600 psi for cardiac scans)
DSA series: Ultra-high pressure up to 1200 psi for angiography
MRI series: 200 psi for low-pressure applications
Q4. With various syringe models available, how can I choose the right one for my device?
A:
Our sales team offers matching support to ensure accurate model selection.
WEGO provides a clear department-based compatibility guide:
CT: 24 models (e.g., CT-MS200 for Medrad Stellant)
DSA: 5 models (e.g., DSA-MM150 for MEDRAD MARK V)
MRI: 6 models (e.g., MRI-ME60/110 for Medrad Solaris)