CSSD Center
Sterile Area Solutions
The sterile area is the final and most critical stage within the Central Sterile Supply Department (CSSD), where sterilized medical devices are inspected, packaged, labeled, and released for clinical use. Any deviation at this stage may compromise sterilization outcomes and patient safety.
The sterile area is the final and most critical stage within the Central Sterile Supply Department (CSSD), where sterilized medical devices are inspected, packaged, labeled, and released for clinical use. Any deviation at this stage may compromise sterilization outcomes and patient safety.
WEGO provides a coordinated range of sterile area equipment designed to support standardized workflows, ensure packaging integrity, and maintain sterility until point of use. Our solutions help healthcare facilities improve traceability, reduce manual errors, and comply with international CSSD standards across diverse clinical environments.

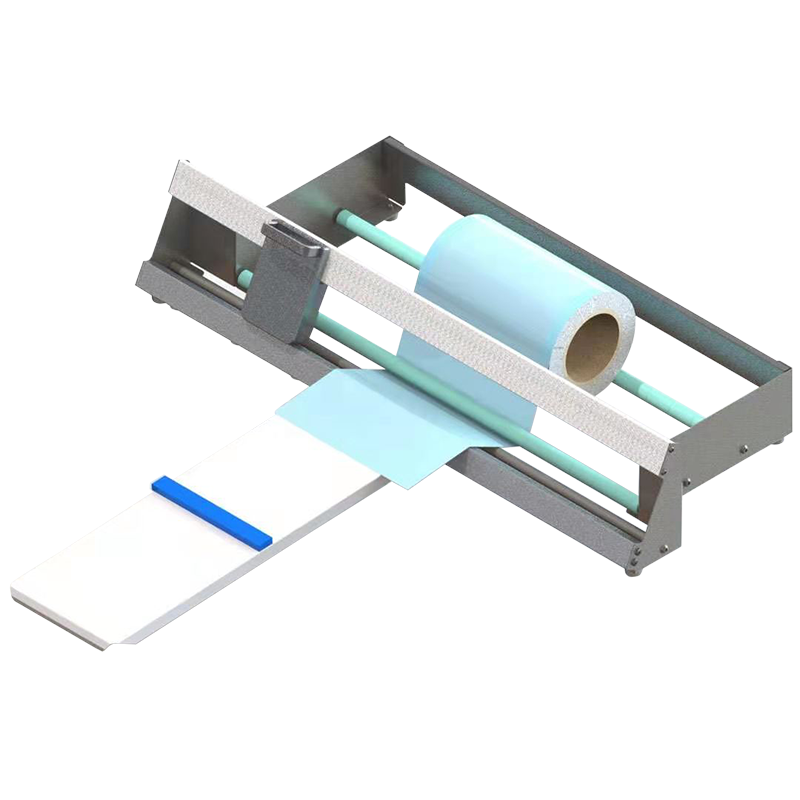
Accurate and efficient cutting of sterilization packaging materials is a key step in standardized packaging procedures. WEGO paper cutters are designed for clean, precise cutting of medical-grade paper and packaging rolls, helping CSSD staff improve efficiency, reduce material waste, and maintain consistent packaging quality.
Rapid biological readers enable fast and reliable interpretation of biological indicators, supporting timely release decisions for sterilized loads. By reducing waiting times compared to traditional incubation methods, WEGO biological readers help CSSD teams improve workflow efficiency while maintaining high standards of sterility assurance and regulatory compliance.

Sterile Area Solutions
The Reliable Sterile Area is the final and most controlled stage of the reprocessing workflow, ensuring that every instrument maintains its sterility and structural integrity after completing validated cycles in the steam sterilizers. Once sterilization is finished, packaged instruments enter a dedicated environment where temperature, humidity, and airflow are carefully regulated to prevent moisture, dust, or secondary contamination. Within this space, standardized shelving layouts and protected placement inside sterile storage cabinets help maintain sterility throughout the storage period. Positive-pressure design, clean zoning, and defined movement pathways further reduce the risk of compromise, ensuring that every sterile package remains secure, traceable, and ready for timely distribution to clinical departments.


Comprehensive Support from the WEGO Global Team
When you partner with WEGO, you gain more than trusted medical products – you gain the strength of the WEGO Global Support Team. We are dedicated to helping you maximize performance, ensure reliability, and deliver better outcomes for patients worldwide.
With 37 years of experience, advanced manufacturing, and a worldwide service network, WEGO provides timely assistance, technical guidance, and tailored solutions. Our team works side by side with you to keep operations running smoothly and efficiently.
At WEGO, our commitment starts before the first delivery and grows throughout our journey together. Your success drives our mission, and your trust fuels our progress.
- Access WEGO Expert Support >>

Q1. What environmental conditions are required to maintain sterility in the Reliable Sterile Area?
A: Temperature, humidity, and positive-pressure airflow must remain within controlled ranges recommended by international guidelines to prevent moisture absorption, dust ingress, and microbial contamination of sterile packages.
Q2. How is the integrity of sterile packaging maintained during storage?
A: Validated sterile packaging materials, proper shelf spacing, and protection from compression, puncture, or excessive handling ensure the sterility maintenance of each pack throughout its storage life.
Q3. What shelving and storage systems are recommended for sterile items?
A: Open or closed sterile storage cabinets, corrosion-resistant shelving, and adjustable modular racks are preferred to maintain airflow, reduce dust accumulation, and protect sterile packs from physical damage.
Q4. What standards and regulatory frameworks do WEGO’s CSSD solutions comply with?
A: WEGO’s CSSD equipment and workflows comply with international standards such as ISO 15883 for washer-disinfectors, ISO 17665 for moist heat sterilization, EN 13060/285 for steam sterilizers, and relevant ISO/CE requirements for material compatibility, bioburden control, and process validation. Compliance ensures consistency, traceability, and global interoperability with hospital infection control systems.
Q5. What factors determine whether instruments should undergo steam sterilization or low-temperature sterilization?
A: Selection depends on material compatibility, heat sensitivity, moisture tolerance, and manufacturer IFU requirements. Stainless steel and most general surgical instruments are steam-compatible, while optical devices, plastic polymers, electronics, and heat-sensitive materials typically require low-temperature plasma sterilization.