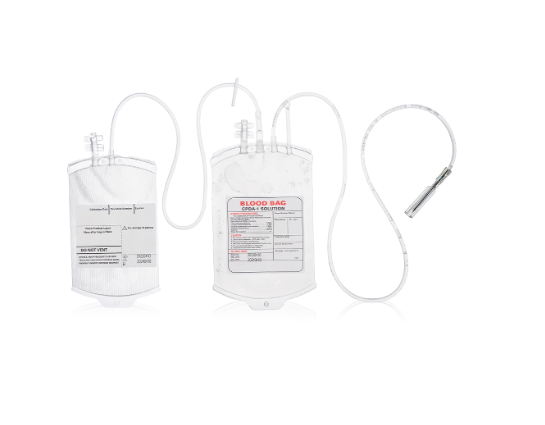
Importing medical consumables from China is no easy task – it requires both reliable products and compliance with international standards. As WEGO Medical, a medical device manufacturer with years of industry experience, we have established a systematic import management process that ensures full control and traceability at every step. We not only produce high-quality medical consumables such as blood bags in-house, but also maintain a rigorous supplier partnership system to consistently provide safe and compliant products to healthcare institutions worldwide.
Choosing the Right Supplier
Choosing a reliable disposable medical products manufacturer is just the beginning. Once a supplier is identified, we conduct in-depth verification of all applicable certifications—such as ISO, CE, and FDA—to ensure alignment with international regulatory requirements. We coordinate closely with customs authorities to prepare accurate documentation, including commercial invoices, product specifications, and safety certificates. Prior to shipment, independent quality inspections are carried out to verify that each batch meets stringent performance and safety criteria. Throughout transportation, our logistics team monitors the process in real time to prevent delays or damage, guaranteeing that products arrive safely and on schedule. This end-to-end control allows us to maintain an efficient, transparent, and fully compliant import process.
Understanding Regulatory Compliance
Regulatory compliance is critical to product safety, legal approval, and patient protection. Medical devices such as double blood bags must satisfy both national and international standards—covering technical documentation, labeling, packaging specifications, and instructions for use—to ensure correct handling and clinical safety. Adherence to CE marking, ISO standards, and local health authority guidelines helps mitigate risks during shipping, storage, and end use. By reviewing these requirements early in the import process, we facilitate smoother customs clearance and give healthcare providers confidence in the sterility, quality, and safety of our products. Complete and accurate documentation also ensures traceability, simplifies audits, and enables swift resolution of potential issues. At WEGO Medical, we apply this regulatory insight to anticipate challenges, prevent delays, and verify that every product meets the highest clinical standards.
Conclusion: Maintaining Quality and Operational Efficiency
Importing medical consumables from China requires meticulous planning, regulatory expertise, and trusted partnerships. By choosing WEGO Medical as your manufacturing and import partner, you gain access to a consistent supply of high-quality double blood bags and other essential medical consumables. Our step-by-step methodology—spanning supplier evaluation, compliance verification, and logistics coordination—ensures safe, effective, and timely delivery to healthcare providers worldwide. This structured and reliable process ultimately strengthens patient care, enhances operational efficiency, and builds confidence across the medical supply chain.